Regulation of Chromatin Structure in the Germ Line

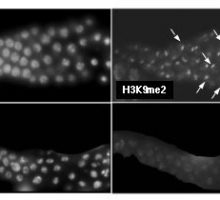
Meiotic silencing of unpaired chromatin is a widespread phenomenon that has been described in many vertebrate and invertebrate organisms. In C. elegans, as in other species, unpaired chromosomes accumulate a high level of repressive chromatin modifications that are not observed on synapsed chromosomes. For example, the male X chromosome accumulates a high level of histone H3 lysine 9 dimethylation (H3K9me2), as do hermaphrodite Xs that fail to synapse due to mutation (see figure). This widely conserved histone modification is associated with assembly of closed chromatin structure and transcriptional repression. Our goals are to understand the mechanisms by which unpaired chromatin is recognized and silenced during meiosis and to identify the chromosomal targets of meiotic silencing.
We have demonstrated that a branch of the C. eleganssmall RNA network functions in regulating meiotic H3K9me2 accumulation and distribution. Components of this pathway include the RNA-directed RNA polymerase, EGO-1, and the Argonaute/Slicer protein, CSR-1. MET-2 is the histone methyltransferase responsible for H3K9me2 accumulation in the germ line. We are working to understand how activity of the EGO-1/CSR-1 small RNA pathway influences MET-2 activity to direct it toward unsynapsed chromosomes.
C. elegans provides an unparalleled system for studying the mechanism of meiotic silencing in the context of germline development. The C. elegans germ line has proven an excellent model for studying genetic regulation and developmental questions. Extensive genetic tools available in C. elegans allow us study the meiotic silencing machinery as well as address larger questions about the role of meiotic silencing in germline development and function. One focus of our current work is to identify additional factors acting together with MET-2 and/or the EGO-1/CSR-1 pathway to regulate H3K9me2 accumulation. Another focus is to identify the sites where H3K9me2 modifications are deposited on unpaired chromosomes. By understanding the mechanism and targets of H3K9me2 accumulation, we will be in a position to investigate the developmental implications of this process. We expect our results to facilitate the study of meiotic silencing in more complex animals, including mammals.
Selected Related Publications:
Guo, Y., B. Yang, Y. Li, X. Xu, and E.M. Maine (2015) Enrichment of H3K9me2 on unsynapsed chromatin in Caenorhabditis elegans does not target de novo sites. G3 5:1865-1878.
Van Wynsberghe, P.M., and E.M. Maine (2012) Epigenetic control of germline development. Advances in Experimental Medicine and Biology, vol 757, in press. Editor: T. Schedl. Publisher: Springer Verlag.
Maine, E.M. (2010) Meiotic silencing in Caenorhabditis elegans. International Review of Cell and Molecular Biology 282, 91-134. [Maine_Meiotic Silencing_2010]
She, X., X. Xu, A. Fedotov, W.G. Kelly, nd E.M. Maine (2009) Regulation of heterochromatin assembly during C. elegans meiosis by components of a small RNA-mediated pathway. PLoS Genetics 5(8): e1000624. doi:10.1371/journal.pgen.1000624 [She_etal_2009]
Maine, E.M., J. Hauth, T. Ratliff, V.E. Vought, X. She, and W.G. Kelly. (2005) EGO-1, a putative RNA-dependent RNA polymerase, is required for heterochromatin assembly on unpaired chromosomes duringC. elegans meiosis. Current Biology, 15, 1972-1978. [Maine_etal_05]
* This paper was cited by the Faculty of 1000.
Vought, V.E., M. Ohmachi, M-H. Lee, and E.M. Maine (2005) EGO-1, a putative RNA-directed RNA polymerase, promotes germline proliferation in parallel with GLP-1/Notch signaling and regulates the spatial organization of nuclear pore complexes and germline P granules in C. elegans. Genetics, 170, 1121-1132. [Vought_etal_05]
Smardon, A., J. M. Spoerke, S.C. Stacey, M.E. Klein, N. Mackin, and E.M. Maine (2000) EGO-1 is related to RNA-directed RNA polymerase and functions in germline development and RNA interference in C. elegans. Current Biology 10, 169-178. [SMARDON ETAL_2000]
*This paper was featured in Science Editor’s Choice and Current Biology Dispatch reviews.
Qiao, L., J.L. Lissemore, P. Shu, A. Smardon, M. Gelber, and E.M. Maine (1995) Enhancers of glp-1, a gene required for cell-signaling in C. elegans, define a set of genes required for germline development.Genetics 141, 551-569. [QIAO ETAL_1995]
