Maine Lab Current Areas of Research
Balancing Germ Cell Renewal and Differentiation
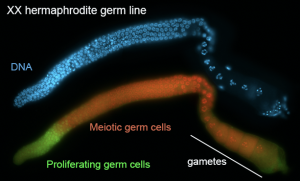 The C. elegans germline forms gametes throughout the adult life of the animal. This process relies on a population of proliferating germ cells that are located at one end of the gonad. From this stem cell population, daughter cells enter meiosis and form gametes. A similar situation is found in many animal tissues, where a population of stem cells allows the continual regeneration of differentiated cells. Our research goal is to understand the mechanisms regulating the balance between germline proliferation and meiosis. C. elegans germline stem cells are maintained by an inductive signal from the somatic gonad. The signal is mediated by Notch-type signaling, a widely conserved signaling mechanism that regulates cell proliferation and/or differentiation in diverse animal species. Nutritional and other inputs work in parallel with GLP-1/Notch signaling to influence the size of the stem cell population. These proliferative inputs are counterbalanced by developmental regulators that move germ cells into meiosis, allowing them to eventually form gametes. We have identified several regulators of germline proliferation, including positive regulators of Notch signaling and proteins working in parallel with Notch signaling. We are working to understand how diverse regulatory pathways are controlled and integrated to maintain a balance between germ cell renewal and gamete formation.
The C. elegans germline forms gametes throughout the adult life of the animal. This process relies on a population of proliferating germ cells that are located at one end of the gonad. From this stem cell population, daughter cells enter meiosis and form gametes. A similar situation is found in many animal tissues, where a population of stem cells allows the continual regeneration of differentiated cells. Our research goal is to understand the mechanisms regulating the balance between germline proliferation and meiosis. C. elegans germline stem cells are maintained by an inductive signal from the somatic gonad. The signal is mediated by Notch-type signaling, a widely conserved signaling mechanism that regulates cell proliferation and/or differentiation in diverse animal species. Nutritional and other inputs work in parallel with GLP-1/Notch signaling to influence the size of the stem cell population. These proliferative inputs are counterbalanced by developmental regulators that move germ cells into meiosis, allowing them to eventually form gametes. We have identified several regulators of germline proliferation, including positive regulators of Notch signaling and proteins working in parallel with Notch signaling. We are working to understand how diverse regulatory pathways are controlled and integrated to maintain a balance between germ cell renewal and gamete formation.
Relevant papers: Lissemore et al. (2018), Safdar et al. (2016), Fox et al. (2011), She et al. (2009), Liu and Maine (2007), Vought et al. (2005), Maine et al. (2004), Qiao et al. (1995)
The Role of RNA Uridylation in Maintaining Germ Cell Identity and Viability
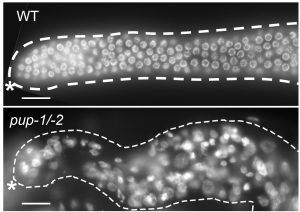
The C. elegans, germline is established in the early embryo and maintained throughout larval development and adulthood. Once the germline is established, several mechanisms are known to help to maintain its distinct identity. We found that one such mechanism involves enzymes called poly(U) polymerases (PUPs) that modify RNAs by adding uridine to the 3’ end. The germline does not express the correct pattern of genes in animals that are mutant for PUP-1 and PUP-2 and raised at high temperature. In these mutants, many germ cells die by apoptosis, and gamete formation is disrupted. We are working now to identify the RNAs that are modified by each PUP and to understand how the 3’ modification influences their stability and function.
Relevant papers: Vieux et al. (2021), Li et al. (2021), Li and Maine (2018)
Chromatin Structure in the Germ Line
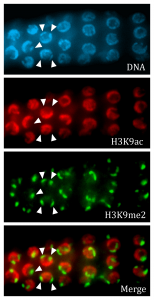 Gene expression is modulated by chromatin structure, including post-translational modifications of histone proteins that promote or limit transcription. Histone modifications are dynamic during germline development, with distinct patterns observed during proliferation, meiosis, and in maturing gametes. We found that the pattern of histone modifications in the C. elegans germ line during meiosis and sperm formation depends, in part, on the small RNA network that is active in the germ line. We are working to understand how histone-modifying enzymes are targeted during development and the role that small RNA-mediated pathways play in this process.
Gene expression is modulated by chromatin structure, including post-translational modifications of histone proteins that promote or limit transcription. Histone modifications are dynamic during germline development, with distinct patterns observed during proliferation, meiosis, and in maturing gametes. We found that the pattern of histone modifications in the C. elegans germ line during meiosis and sperm formation depends, in part, on the small RNA network that is active in the germ line. We are working to understand how histone-modifying enzymes are targeted during development and the role that small RNA-mediated pathways play in this process.
Relevant papers: Li et al. (2021), Yang et al. (2019), Mutlu et al. (2018), Guo et al. (2015), Van Wynsberghe & Maine (2012), Maine (2010), She et al. (2009), Maine et al. (2005), Vought et al. (2005), Smardon et al. (2000)
